
For UCalgary Researchers
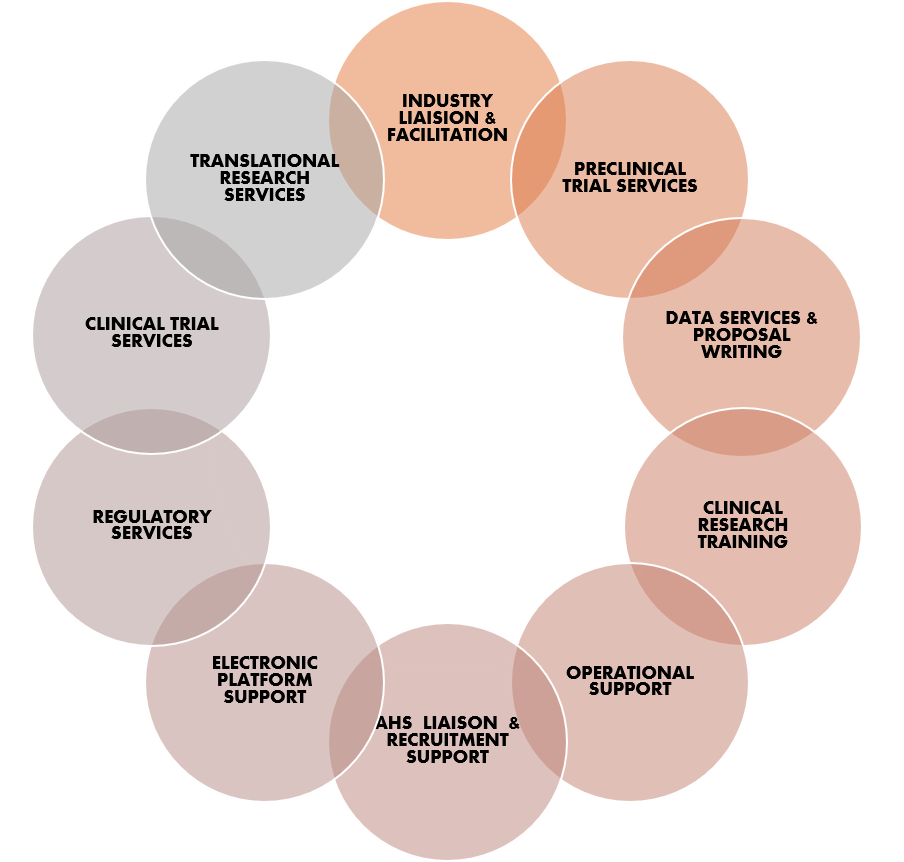
University of Calgary researchers have access to a full suite of support services that span the entire clinical trial lifecycle. These services are delivered by a network of support teams, offices and groups. Support is available for both industry-initiated and investigator-initiated studies.
Researchers interested in bringing their ideas to market can access a range of commercialization, pre- and post-market resources, including entrepreneurial and business development programming, mentorship opportunities, and more.
If you are looking resources or services to support your clinical trial, the best starting point is to explore the research resources provided by Research at UCalgary and the Cumming School of Medicine (CSM).
If you are unsure of where to start or have any questions, we can help. Simply contact us to get started.
Common questions and answers
Here, you'll find common questions asked by researchers conducting clinical trials at the University of Calgary. If you have a question that isn't listed, please contact us.
Through a network of support offices and teams, the University of Calgary offers a full suite of clinical trial services. From study start-up to commercialization, researchers can receive support to address the regulatory, operational and financial challenges that are associated with trials. This can include:
Study Start-up
The Calgary Centre for Clinical Research (CCCR) offers start-up services related to sponsored clinical trials, including:
- Site selection and trial start-up support
- Compiling the sponsor’s requested information for a site selection visit, facilitating the meeting and ensuring that follow-up requirements are met
- Coordinating activities such as CDA/CTA and budget submission, completion of feasibility questionnaires and regulatory documents, initial REB submission, and AHS approval requests
- Advising on available clinical research services and assisting clinical researchers with accessing clinical research services as needed
- Acting as a point of contact for questions regarding research facilities, resources and requirements
Industry Liaison services
The Calgary Centre of Clinical Research offers industry liaison services related to sponsored clinical trials, including:
- Acting as the first point of contact for internal and external stakeholders who have inquiries about conducting clinical trials at the University
- Communicating with pharmaceutical companies and contract research organizations about upcoming sponsored clinical trial opportunities
- Connecting appropriate investigators with sponsored clinical trial opportunities
- Offering support during the site selection phase
Regulatory and compliance guidance
Compliance-related services for clinical researchers offered through the University’s QA and Regulatory Compliance Program. Support is provided in the following areas:
- Conducting internal regulatory file reviews
- Guiding researchers through the regulations and guidelines governing research projects (e.g. University policies, REB SOPs, TCPS2, ICH-GCP, Health Canada, US FDA regulations, etc.)
- Supporting researchers who are undergoing inspections from regulatory bodies, such as Health Canada and the FDA
- Implementing and maintaining N2 Standard Operating Procedures for clinical trials conducted at the University of Calgary.
- Trial registration with ClinicalTrials.gov
Budget Support
The Calgary Centre for Clinical Research budget team is skilled in reviewing and developing accurate budgets for both industry sponsored and grant funded trials. The team negotiates trial budgets and payments terms with industry sponsors, ensuring fiscally sound projects and preventing cost overruns.
Legal Services
The Cumming School of Medicine’s Legal Team (CSM Legal) provides legal review of research contracts and agreements for clinical trials. Assistance is available with drafting, negotiation and execution of trial contracts.
The University of Calgary is home to numerous, state-of-the-art labs and facilities that help empower research across a range of therapeutic areas. For a non-exhaustive listing, visit the Labs and Facilities page.
Pharmacy needs of University of Calgary Researchers are supported by the AHS Research Pharmacy at U of C locations as well as AHS hospitals and health care facilities located in Calgary. The AHS research pharmacy has GCP trained pharmacists, pharmacy technicians and a pharmacy coordinator with knowledge of the regulatory requirements for clinical research in Canada and experience in receiving, storing, preparing and managing study drug accountability. Pharmacy equipment includes:
- Temperature monitored storage (room temperature, walk-in cooler, standard freezer and ultra-low freezer)
- Class 7 sterile and ante room
- Class 2, type B2 biosafety cabinet
- A -80 C non-cycling freezer for drug storage
The Research Pharmacy can be reached at pharmacy.research@albertahealthservices.ca and shipments can be received at:
AHS Research Pharmacy
TRW Building, 5th floor
3280 Hospital Dr. NW
W21C offers capacity building and staffing services to researchers including trained clinical trial coordinators and nurses.
Where Can I find Systems and Data Services for Investigator Initiated Trials?
The Clinical Research Unit offers systems and data services including:
-
- Electronic Data Capture Systems (REDCap)
- Methods and Analytics Support (study design, randomization, and statistical analysis).
- Custom Software Development (custom clinical trial databases)
In an effort to assist researchers with pre- and post-awards for investigator initiated external research funding, the Office of the Associate Dean Research (OADR) now has an office devoted to Grant Development and Research Facilitation. This team is your first point of contact for support with external funding agency applications.
IMPACT is a program for new ventures in the life sciences or biomedical industries who seek to commercialize their health-related inventions, conduct clinical trials, and secure regulatory approval. The IMPACT Venture Navigators work with the venture to design, support, execute, and report on their clinical trials, including connecting the venture with a Principal Investigator.
The Calgary Centre of Clinical Research offers industry liaison services related to sponsored clinical trials including:
- First Point of contact for internal and external stakeholders who have inquiries pertaining to the conduct of clinical trials at the University of Calgary
- Communication with pharmaceutical companies and contract research organizations about upcoming sponsored clinical trial opportunities
- Matching sponsored clinical trial opportunities with investigators
- Support during the site selection phase of a new trial
In addition, the CCCR provides a site information presentation that can be used when liaising with industry.
Innovate Calgary is a full-service organization offering technology transfer and business incubator services to researchers, entrepreneurs and businesses within the advanced technology sector. Located in the Alastair Ross Technology Centre in the Research Park adjacent to the main campus of the University of Calgary, the organization supports the technology community and is dedicated to the growth of technology commercialization. In cooperation with Research Services, Innovate Calgary works to help researchers at the University of Calgary to bridge the gap between discovery and innovation through commercialization of the intellectual property emerging from their research. Services include expert advice and assistance with:
- Intellectual property (IP) protection and strategy (patent, copyright and trademark registration)
- Marketing and licensing IP
- Company creation and incubation
- Analysis of technologies for commercial potential
- Business and technical advice and skills workshops
- Mitigation of financial and resource risks of technology commercialization, company creation and investment attraction

Let us help.
Have questions about the clinical trial and commercialization resources available to UCalgary researchers? We can help.
